Our news
Home /
 Transition to the single market of EEU, top – 6 of hot questions
21.10.2019
Read more
Transition to the single market of EEU, top – 6 of hot questions
21.10.2019
Read more
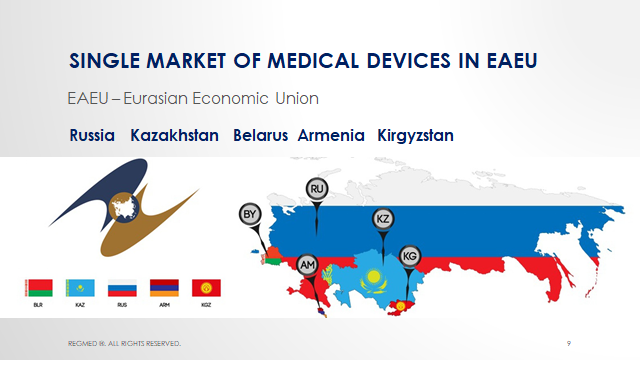 Registration of medical devices. Hot news. Date of mandatory re-registration of medical devices according to EAEU standards will be shifted
18.08.2019
Read more
Registration of medical devices. Hot news. Date of mandatory re-registration of medical devices according to EAEU standards will be shifted
18.08.2019
Read more
 RegMed company took part in the conference of UNIDI Unione Nazionale Industrie Dentarie Italiane – Italian Dental Industries Association, Milan, 9 July 2019
06.08.2019
Read more
RegMed company took part in the conference of UNIDI Unione Nazionale Industrie Dentarie Italiane – Italian Dental Industries Association, Milan, 9 July 2019
06.08.2019
Read more
 We invite you to take part in master-class “Russian GMP certificate. Procedure. Key points. Recommendations”
27.03.2019
Read more
We invite you to take part in master-class “Russian GMP certificate. Procedure. Key points. Recommendations”
27.03.2019
Read more
 Vladimir Putin issued an instruction to shorten the time frame for conducting expert examinations of anaesthetic drugs
25.08.2017
Read more
Vladimir Putin issued an instruction to shorten the time frame for conducting expert examinations of anaesthetic drugs
25.08.2017
Read more
 Draft laws on the introduction of a system for monitoring the movement of medicines have been submitted to the State Duma
20.07.2017
Read more
Draft laws on the introduction of a system for monitoring the movement of medicines have been submitted to the State Duma
20.07.2017
Read more
 Safety profile of registered generic medicines to be increased
13.06.2017
Read more
Safety profile of registered generic medicines to be increased
13.06.2017
Read more
 ЕАEU states’ national markets for the circulation of medicines start working in a unified format
10.05.2017
Read more
ЕАEU states’ national markets for the circulation of medicines start working in a unified format
10.05.2017
Read more
 The EEC and WHO have begun preparations for the recognition of the EAEU Pharmacopoeia as a regional standard
05.05.2017
Read more
The EEC and WHO have begun preparations for the recognition of the EAEU Pharmacopoeia as a regional standard
05.05.2017
Read more
 Duty rates set for registration of biomedical cell products, medicines and medical products in the Eurasian Economic Union common market
09.03.2017
Read more
Duty rates set for registration of biomedical cell products, medicines and medical products in the Eurasian Economic Union common market
09.03.2017
Read more
 Boiron calls for the effectiveness of homeopathy to judged on the results of clinical practice
21.02.2017
Read more
Boiron calls for the effectiveness of homeopathy to judged on the results of clinical practice
21.02.2017
Read more
 The Russian Health Ministry has decided on package volumes for alcohol-containing medicines
08.02.2017
Read more
The Russian Health Ministry has decided on package volumes for alcohol-containing medicines
08.02.2017
Read more